The concept required to solve this problem is that related to the Isobaric process. Isobaric process is understood as the process in which changes occur at constant pressure. From the first law of thermodynamics this can be expressed as,
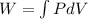
Here,
P = Pressure
dV = Differential of Volume
As the Pressure is constant we have,
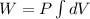
![W = P \big [ V \big ]^(V_f)_(V_i)](https://img.qammunity.org/2021/formulas/physics/college/3rc0fm9gqcqbds0olxwezd2wpvyu0vcayh.png)
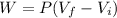
Replacing
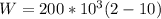
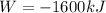
Therefore the correct answer is A.