Answer: The complete ionic equation is written below.
Step-by-step explanation:
Complete ionic equation is defined as the equation in which all the substances that are strong electrolyte are present in an aqueous are represented in the form of ions.
The balanced molecular equation for the reaction of lead (II) nitrate and potassium sulfate follows:
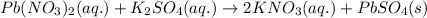
The complete ionic equation for the above equation is:
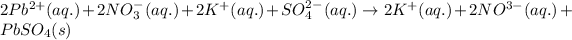
Hence, the complete ionic equation is written above.