Answer: The equilibrium constant for the reaction is 2.47
Step-by-step explanation:
We are given:
Initial moles of ammonia gas = 29.0 moles
Equilibrium moles of nitrogen gas = 13.0 moles
Volume of the tank = 7.50 L
Molarity is calculated by using the formula:
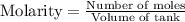
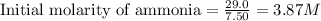
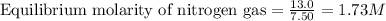
The chemical equation for the decomposition of ammonia follows:
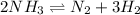
Initial: 3.87
At eqllm: 3.87-2x x 3x
Evaluating the value of 'x'
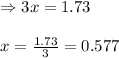
So, equilibrium concentration of ammonia = (3.87 - 2x) = [3.87 - 2(0.577)] = 2.716 M
Equilibrium concentration of nitrogen gas = x = 0.577 M
The expression of
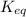 for above equation follows:
for above equation follows:
![K_(eq)=([N_2][H_2]^3)/([NH_3]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/3no27zk1mgr2769n6lpki5sg7wrif58a7g.png)
Putting values in above equation, we get:
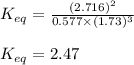
Hence, the equilibrium constant for the reaction is 2.47