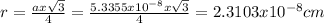Answer:
lattice parameter = 5.3355x10^-8 cm
atomic radius = 2.3103x10^-8 cm
Step-by-step explanation:
known data:
p=0.855 g/cm^3
atomic mass = 39.09 g/mol
atoms/cell = 2 atoms
Avogadro number = 6.02x10^23 atom/mol
a) the lattice parameter:
Since potassium has a cubic structure, its volume is equal to:
v = [(atoms/cell)x(atomic mass)/(p)x(Avogadro number)]
substituting values:
v =[(2)x(39.09)/(0.855x6.02x10^23)]=1.5189x10^-22 cm^3
but as the cell volume is
a^3 =v
![a=\sqrt[3]{v}=\sqrt[3]{1.5189x10^(-22) } = 5.3355x10^-8](https://img.qammunity.org/2021/formulas/chemistry/high-school/yhu4w16bohnpr7dni9kommv8uy37suv1f9.png) cm
cm
for a BCC structure, the atomic radius is equal to