Answer:
The pH is 7.54
Step-by-step explanation:
The Henderson - Hasselbalch equation states that for a buffer solution which consists of a weak acid and its conjugate base, the buffer pH is given by:
pH
![=pk_(a) +log(([conjugate base])/([weakacid]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/r158vh6ivj3ubjuu89ze1ckuwvf3yt6y2b.png)
pkₐ is for the acid
In this case, the buffer hypochlorous acid HClO is a weak acid, and its conjugate base is the hypochlorite anion ClO⁻ is delivered to the solution via sodium hypochlorite NaClO .
NaCIO = 0.200 M
HCIO = 0.200 M
pkₐ = -log₁₀ kₐ = -log₁₀ (2.9 × 10⁻⁸) = 7.54
∴pH =
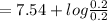 = 7.54
= 7.54