This is an incomplete question, here is a complete question.
The rearrangement of methyl isonitrile (CH₃NC) to acetonitrile (CH₃NC) is a first-order reaction and has a rate constant of 5.11 × 10⁻⁵ s⁻¹ at 472 K. If the initial concentration of CH₃NC is 3.00 × 10⁻² M :
How many hours will it take for the concentration of methyl isonitrile to drop to 14.0 % of its initial value?
Answer : The time taken will be, 10.7 hours
Explanation :
Expression for rate law for first order kinetics is given by:
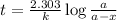
where,
k = rate constant =
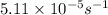
t = time passed by the sample = ?
a = let initial amount of the reactant = 100
a - x = amount left after decay process = 14 % of 100 = 14
Now put all the given values in above equation, we get
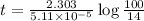
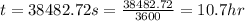
Therefore, the time taken will be, 10.7 hours