The question is incomplete, complete question is:
Carbon tetrachloride can be produced by this reaction:
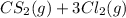 ⇌
⇌
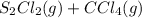
Suppose 1.1 mol and 3.3 mol are placed in a 1.00-L flask and the flask is sealed. After equilibrium has been achieved, the mixture contains 0.82 mol .
Calculate
 .
.
Answer:
The value of the
 of the reaction is 4.05.
of the reaction is 4.05.
Step-by-step explanation:
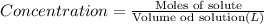
Initial concentration of
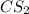 :
:
![[CS_2]=(1.1 mol)/(1 L)=1.1 M](https://img.qammunity.org/2021/formulas/chemistry/college/9zh0hb08kc5f8hh604ggsyt7m1omd6m2ud.png)
Initial concentration of
 :
:
![[Cl_2]=(3.3mol)/(1 L)=3.3M](https://img.qammunity.org/2021/formulas/chemistry/college/abjtvfodjqs9n6clxo576n2etqcvv1e6kx.png)
Equilibrium concentration of
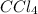 :
:
![[CCl_4]=(0.82 mol)/(1 L)=0.82 M](https://img.qammunity.org/2021/formulas/chemistry/college/4g2771b95sf033b6mw9t8asx97nc46k4f8.png)
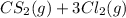 ⇌
⇌
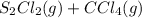
initially :
1.1 M 3.3 M 0 0
At equilibrium
(1.1-0.82) M (3.3-3 × 0.82) M 0.82 M 0.82 M
0.28 M 0.84 0.82 0.82
The expression of equilibrium constant
 is given by :
is given by :
![K_c=([S_2Cl_2][CCl_4])/([CS_2][Cl_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/sxyggu0lx42fuwxpnf3tso8tudifvac17z.png)
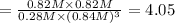
The value of the
 of the reaction is 4.05.
of the reaction is 4.05.