Answer: The corresponding value of
 for this reaction at 84.5°C is 0.00232
for this reaction at 84.5°C is 0.00232
Step-by-step explanation:
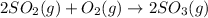
Relation of with is given by the formula:
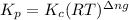
where,
= equilibrium constant in terms of partial pressure = ?
 = equilibrium constant in terms of concentration = 0.0680
= equilibrium constant in terms of concentration = 0.0680
R = Gas constant =
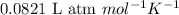
T = temperature =
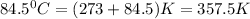
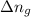 = change in number of moles of gas particles =
= change in number of moles of gas particles =
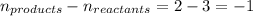
Putting values in above equation, we get:
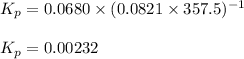
Thus the corresponding value of
 for this reaction at 84.5°C is 0.00232
for this reaction at 84.5°C is 0.00232