Step-by-step explanation:
It is known that at the equivalence point,
Equivalent of acid = equivalent of base ......... (A)
and, Equivalent =
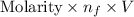
Therefore, equivalent of acid is calculated as follows.
Equivalent of acid =
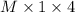 .......... (1)
.......... (1)
Equivalent of base =
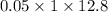 ............ (2)
............ (2)
Hence, using equation (A) we will calculate the concentration as follows.
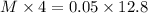
M = 0.16 M
Thus, we can conclude that concentration of the unknown weak acid solution 0.16 M.