The question in incomplete, complete question is;
Determine the theoretical yield:
Excess aqueous copper(II) nitrate reacts with aqueous sodium sulfide to produce aqueous sodium nitrate and copper(II) sulfide as a precipitate. In this reaction 469 grams of copper(II) nitrate were combined with 156 grams of sodium sulfide to produce 272 grams of sodium nitrate.
Answer:
The theoretical yield of sodium nitrate is 340 grams.
Step-by-step explanation:
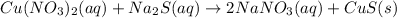
Moles of copper(II) nitrate =
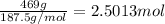
Moles of sodium sulfide =
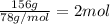
According to reaction, 1 mole of copper (II) nitrate reacts with 1 mole of sodium sulfide.
Then 2 moles of sodium sulfide will react with:
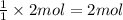 of copper (II) nitrate
of copper (II) nitrate
As we can see from this sodium sulfide is present in limiting amount, so the amount of sodium nitrate will depend upon moles of sodium sulfide.
According to reaction, 1 mole of sodium sulfide gives 2 mole of sodium nitrate, then 2 mole of sodium sulfide will give:
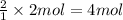 sodium nitrate
sodium nitrate
Mass of 4 moles of sodium nitrate :
85 g/mol × 4 mol = 340 g
Theoretical yield of sodium nitrate = 340 g
The theoretical yield of sodium nitrate is 340 grams.