Step-by-step explanation:
(a) First, we will calculate the charge of sodium ions as follows.
q = ne
=
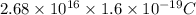
=
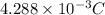
Now, charge of chlorine ions is calculated as follows.
q' = ne
=
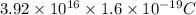
=
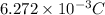
Hence, the current will be calculated as follows.
i =
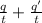
=
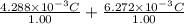
=
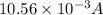
= 10.56 mA
Therefore, current passing between the electrodes is 10.56 mA.
(b) Since, positive ions are moving towards the negative electrode. And, current is the flow of ions or electrons therefore, the direction of current is towards the negative electrode.