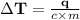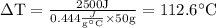Temperature change would be 112.6° C.
Step-by-step explanation:
We can find the amount or heat absorbed or emitted during any reaction by finding the product of their mass, specific heat, and change in temperature of the metal.
Mass of the iron, m = 50.0 g
Amount of heat absorbed, q = 2500 J
Change in temperature, ΔT = ?
Specific heat of Iron, C = 0.444 J/g °C
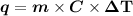
Plugin the values and rearrange the equation to get the change in temperature as,