Answer: Option (A) is the correct answer.
Step-by-step explanation:
Chemical equation for the given reaction is as follows.
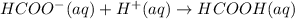
And, the expression to calculate pH of this reaction is as follows.
pH =
![pk_(a) + log ([HCOO^(-)])/([HCOOH])](https://img.qammunity.org/2021/formulas/chemistry/college/1n1yye6gp3gj0n2w135f2nmd1t7unshkj8.png)
As the concentration of
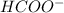 is directly proportional to pH. Hence, when there occurs a decrease in the pH of the solution the
is directly proportional to pH. Hence, when there occurs a decrease in the pH of the solution the
![[HCOO^(-)]](https://img.qammunity.org/2021/formulas/chemistry/college/xy5wqnb13kchs9tu70r3qclm3pol7g0rt0.png) will also decrease.
will also decrease.
Thus, we can conclude that the statement, HCOO will accept a proton from HCl to produce more HCOOH and
 , best supports the student's claim.
, best supports the student's claim.