Answer:
Concentration of sodium nitrate solution is 0.636 mmol/L
Step-by-step explanation:
We know,
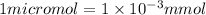
So,
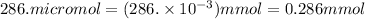
Concentration in mmol/L is defined as number of mmol of solute dissolved in 1000 mL of solution
Here solute is
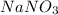
Total volume of solution = Total volume of volumetric flask = 450. mL
Hence concentration of
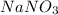 solution =
solution =
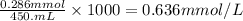
So, concentration of sodium nitrate solution is 0.636 mmol/L