Answer:
20.44% is the percentage of dimethylphthalate in the sample.
Step-by-step explanation:
Molarity of NaOH =
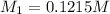
Volume of NaOH consumed in back titration =
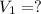
Molarity of HCl =
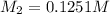
Volume of HCl =
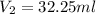

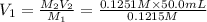
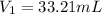
Volume of NaOH used in hydrolysis of ester = 50.00 mL - 33.21 mL = 16.79 mL[/tex]
Moles of NaOH in 16.79 ml of 0.1215 M solution : n
Volume of solution = 16.79 mL = 0.01679 L ( 1mL=0.001 L)
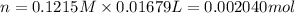
1 mole of NaOH has 1 mole of hydroxide ions than 0.002040 moles of NaOH will have :
1 × 0.002040 mol = 0.002040 mol of hydroxide ions
Moles of hydroxide ions = 0.002040 mol

According to reaction, 2 moles of hydroxide ion reacts with 1 mole of dimethylphthalate , then 0.002040 moles of hydroxide ion swill react with ;
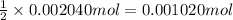 of dimethylphthalate
of dimethylphthalate
Mass of 0.001020 moles of dimethylphthalate :
194 g/mol × 0.001020 mol = 0.1979 g
Mass of sample = 0.9679 g
Mass of dimethylphthalate = 0.1979 g
Percentage of dimethylphthalate in sample;
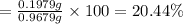
20.44% is the percentage of dimethylphthalate in the sample.