Step-by-step explanation:
It is known that a bond formed due to sharing of electrons is called a covalent bond.
For example, the electronic configuration of hydrogen atom is
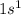 .
.
And, in order to attain stability hydrogen atom will readily combine with another atom to complete its octet. Hence, when it combines with another hydrogen atom then attractive forces increases between the two hydrogen atoms and it forms a
 atom.
atom.
Due to the increase in stability pf both H atoms there will occur a decrease in their potential energy as the atoms will move towards each other.
Thus, we can conclude that when two H atoms are brought together from large distances they can make
 . As the bond beings to form, the force between the atoms becomes more attractive and the potential energy decreases.
. As the bond beings to form, the force between the atoms becomes more attractive and the potential energy decreases.