Answer : The equilibrium partial pressure of Cl₂ at 25°C is, 1.98 atm
Explanation :
For the given chemical reaction:
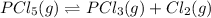
The expression of
 for above reaction follows:
for above reaction follows:
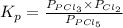
We are given:
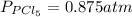
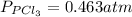
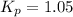
Putting values in above equation, we get:
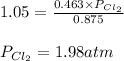
Thus, the equilibrium partial pressure of Cl₂ at 25°C is, 1.98 atm