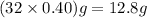Answer:
12.8 g of
 must be withdrawn from tank
must be withdrawn from tank
Step-by-step explanation:
Let's assume
 gas inside tank behaves ideally.
gas inside tank behaves ideally.
According to ideal gas equation-
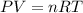
where P is pressure of
 , V is volume of
, V is volume of
 , n is number of moles of
, n is number of moles of
 , R is gas constant and T is temperature in kelvin scale.
, R is gas constant and T is temperature in kelvin scale.
We can also write,
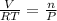
Here V, T and R are constants.
So,
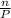 ratio will also be constant before and after removal of
ratio will also be constant before and after removal of
 from tank
from tank
Hence,
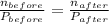
Here,
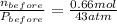 and
and
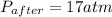
So,
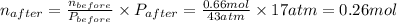
So, moles of
 must be withdrawn = (0.66 - 0.26) mol = 0.40 mol
must be withdrawn = (0.66 - 0.26) mol = 0.40 mol
Molar mass of
 = 32 g/mol
= 32 g/mol
So, mass of
 must be withdrawn =
must be withdrawn =