Answer:
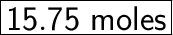
Step-by-step explanation:
Given reaction is:
3X + 2Y → 5C + 4D
In this reaction, we have:
For 3 moles of X, 4 moles of D
So,
4D = 3X
Divide both sides by 4
D = 3X/4
Multiply both sides by 21
21D = 3X/4 × 21
21D = 3*21X/4
21D = 63X/4
21D = 15.75X
So, to produce 21 moles of D, we need 15.75 moles of X.
![\rule[225]{225}{2}](https://img.qammunity.org/qa-images/2023/formulas/biology/high-school/vdifidejf5i8c49g0hg7.png)
Hope this helped!
~AH1807