Answer:
259 mg is the mass of silver chloride required to plate 195 mg of pure silver.
Step-by-step explanation:
Silver chloride contains 75.27 percent silver. It can be concluded that:-
For every 100 mg of silver chloride, 75.27 mg of silver we can get.
So, to produce 195 mg of silver, the amount of silver chloride required can be calculated as:-
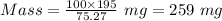
259 mg is the mass of silver chloride required to plate 195 mg of pure silver.