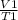 = k
= k
Step-by-step explanation:
The relation between volume, V of gas and Temperature, T of a gas is related by Charles Law.
This law states that the volume of a given amount of gas held at a constant pressure is directly proportional to the Kelvin temperature
Thus,
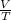 = k
= k
where k is a constant
Therefore,
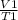 =
=
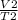 =
=
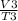 ...
...
This shows, as the volume of a gas goes up, the temperature also goes up and vice-versa.