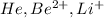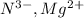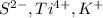Answer : The isoelectronic groups are:
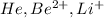
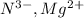
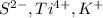
Explanation :
Isoelectronic : It is defined as the compound or molecule having the same number of electrons and the same number of electronic structure.
- The element is helium. The number of electrons are 2.
- The element is beryllium. The number of electrons are 4. The number of electrons in
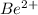 = 4 - 2 = 2
= 4 - 2 = 2 - The element is lithium. The number of electrons are 3. The number of electrons in
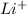 = 3 - 1 = 2
= 3 - 1 = 2 - The element is nitrogen. The number of electrons are 7. The number of electrons in
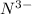 = 7 + 3 = 10
= 7 + 3 = 10 - The element is neon. The number of electrons are 10.
- The element is sulfur. The number of electrons are 16. The number of electrons in
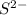 = 16 + 2 = 18
= 16 + 2 = 18 - The element is magnesium. The number of electrons are 12. The number of electrons in
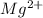 = 12 - 2 = 10
= 12 - 2 = 10 - The element is titanium. The number of electrons are 22. The number of electrons in
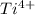 = 22 - 4 = 18
= 22 - 4 = 18 - The element is potassium. The number of electrons are 19. The number of electrons in
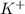 = 19 - 1 = 18
= 19 - 1 = 18
The isoelectronic groups are: