Answer:
Step-by-step explanation:
Pressure (P), volume (V), temperature (T), and number of moles (n) of gases are related.
Use the ideal gas equation:

When volume and temperature of two gases are equal, you can write:
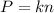
Meaning that the pressure and the number of moles are directly related.
As for the statements:
1) Because the gases have the same mass, they exert the same pressures.
It is false, because the pressure is related to the number of moles of gas and not the mass.
2) Both the volumes and the temperatures of the gases are the same, which means that their pressures must be equal.
False because the number of moles are not equal.
3) There are different numbers of moles in the flasks, meaning that the pressures are different.
True. The number of moles of each gas is calculated dividing the mass by the molar mass, since the masses are equal but the molar masses not, the number of moles are different.
As the number of moles are different, the pressures are different.