Answer:
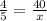
Where x represent the value of interest on this case. And solving for the value of x we have:
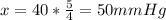
So then the new arterial pressure needs to be now 50 mm Hg to mantain the original level.
And in order to find the concentration we can use a figure called the "O2
-CO2 diagram showing a ventilation-perfusion ratio line. " and when we use this graph to calculate the pressure of Co2 for PO2= 40 mmHg and for Po2=50 mm Hg we got and increase of 0.07 or 7%
So then the final answer for this case would be an increase of 7%
Step-by-step explanation:
For this case we know that a man with normal lungs have an arterial Po2 os 40 mm Hg.
Then we know that this man take an overdose of barbiturates thats halves his aveolar ventilation without changing his metabolism
We also know that the respiratory exchange ratio is 0.8 or 8/10
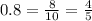
And we want to find hor much does his inspired oxyden concentration % have to increased to return his avelolar Po2 to the original level.
On this case we can apply a proportion rule and we have this:
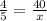
Where x represent the value of interest on this case. And solving for the value of x we have:
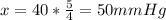
So then the new arterial pressure needs to be now 50 mm Hg to mantain the original level.
And in order to find the concentration we can use a figure called the "O2
-CO2 diagram showing a ventilation-perfusion ratio line. " and when we use this graph to calculate the pressure of Co2 for PO2= 40 mmHg and for Po2=50 mm Hg we got and increase of 0.07 or 7%
So then the final answer for this case would be an increase of 7%