Answer: The vapor pressure of water in the air is 27.58 torr
Step-by-step explanation:
We are given:
Temperature of water in air = 85.0°F
Converting the temperature from degree Fahrenheit to degree Celsius is:
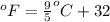
where,
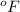 = temperature in Fahrenheit
= temperature in Fahrenheit
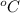 = temperature in centigrade
= temperature in centigrade
So,
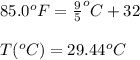
To calculate the final pressure, we use the Clausius-Clayperon equation, which is:
![\ln((P_2)/(P_1))=(\Delta H)/(R)[(1)/(T_1)-(1)/(T_2)]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/uj4tftzxjftyk6uqwm6xdwsj516g82rx28.png)
where,
 = initial pressure which is the pressure at normal boiling point = 760 torr
= initial pressure which is the pressure at normal boiling point = 760 torr
 = final pressure = ?
= final pressure = ?
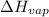 = Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)
= Enthalpy of vaporization = 40.7 kJ/mol = 40700 J/mol (Conversion factor: 1 kJ = 1000 J)
R = Gas constant = 8.314 J/mol K
 = initial temperature =
= initial temperature =
![100^oC=[100+273]K=373K](https://img.qammunity.org/2021/formulas/chemistry/college/3ohy0cydzcvsf08t6t8l841ms10x16dzem.png)
 = final temperature =
= final temperature =
![29.44^oC=[29.44+273]=302.44K](https://img.qammunity.org/2021/formulas/chemistry/middle-school/1p1emp7ctsuwdmvv5gh4anuznh2vh4d485.png)
Putting values in above equation, we get:
![\ln((P_2)/(760))=(40700J/mol)/(8.314J/mol.K)[(1)/(373)-(1)/(302.44)]\\\\P_2=35.72torr](https://img.qammunity.org/2021/formulas/chemistry/middle-school/h579clvk8mwgm6p2bw4uvpmgy9w9lupnam.png)
We are given:
68.0 % of water in the air
This means that 68 grams of water is present in the air
Mass of air = 100 - 68 = 32 g
To calculate the number of moles, we use the equation:

Molar mass of water = 18 g/mol
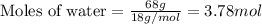
Average molar mass of air = 29 g/mol
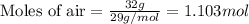
Mole fraction of a substance is given by:
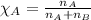

To calculate the mole fraction of substance, we use the equation given by Raoult's law, which is:
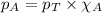
where,
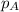 = vapor pressure of water = ?
= vapor pressure of water = ?
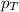 = total pressure = 35.72 torr
= total pressure = 35.72 torr
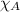 = mole fraction of water = 0.774
= mole fraction of water = 0.774
Putting values in above equation, we get:
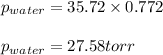
Hence, the vapor pressure of water in the air is 27.58 torr