Answer: alpha decay and beta decay cause the parent nucleus to become a different element.
Step-by-step explanation:
Alpha decay : It is a radioactive decay in which nucleus emits an alpha particle and turns into different atomic nucleus. During alpha decay, an atom loses 2 protons and 2 neutrons.
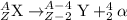
Beta decay is defined as the process in which beta particle is emitted. In this process, a neutron gets converted to a proton and an electron.Thus a different element is produced.
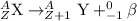
Gamma decay : In this process, the unstable nuclei transformed into stable nuclei by releasing gamma rays along with beta or alpha decay.The same element is produced.
