The question is incomplete, here is the complete question:
An ice calorimeter measures quantities of heat by the quantity of ice melted. How many grams of ice would be melted by the heat released in the complete combustion of 1.60 L of propane gas, measured at 20.0 °C and 735 mmHg? [Hint: What is the standard molar enthalpy of combustion of C₃H₈(g)?]
Answer: The mass of ice that would be melted is 425.52 grams
Step-by-step explanation:
- To calculate the moles of propane, we use the equation given by ideal gas which follows:

where,
P = pressure of the gas = 735 mmHg
V = Volume of the gas = 1.60 L
T = Temperature of the gas =
![20^oC=[20+273]K=293K](https://img.qammunity.org/2021/formulas/chemistry/college/7dd1l57ltqsaiysq0gq719konmiog8fklj.png)
R = Gas constant =
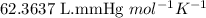
n = number of moles of propane = ?
Putting values in above equation, we get:
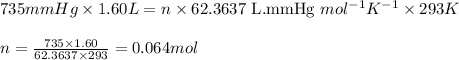
- The equation used to calculate enthalpy change is of a reaction is:
![\Delta H^o_(rxn)=\sum [n* \Delta H_f_((product))]-\sum [n* \Delta H_f_((reactant))]](https://img.qammunity.org/2021/formulas/chemistry/high-school/d42uy6x9fukypqy4uarr52v887yviorkhi.png)
The chemical equation for the combustion of propane follows:
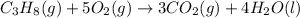
The equation for the enthalpy change of the above reaction is:
![\Delta H_(rxn)=[(3* \Delta H_f_((CO_2(g))))+(4* \Delta H_f_((H_2O(g))))]-[(1* \Delta H_f_((C_3H_8(g))))+(5* \Delta H_f_((O_2(g))))]](https://img.qammunity.org/2021/formulas/chemistry/college/mgd6kuzr8oxvnpzmt5a8uneww5s0kpu974.png)
We are given:
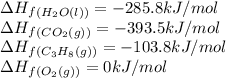
Putting values in above equation, we get:
![\Delta H_(rxn)=[(3* (-393.5))+(4* (-285.8))]-[(1* (-103.8))+(5* (0))]\\\\\Delta H_(rxn)=-2219.9kJ](https://img.qammunity.org/2021/formulas/chemistry/college/y9g0mil472rednedv8c7fzduetl31hi6vv.png)
By Stoichiometry of the reaction:
When 1 mole of propane is combusted, the heat released is 2219.9 kJ
So, when 0.064 moles of propane is combusted, the heat released will be =
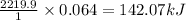
- To calculate the moles of ice, we use the equation:
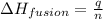
where,
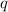 = amount of heat released = 142.07 kJ
= amount of heat released = 142.07 kJ
n = number of moles of ice = ?
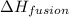 = molar heat of fusion = 6.01 kJ/mol
= molar heat of fusion = 6.01 kJ/mol
Putting values in above equation, we get:
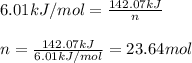
- To calculate the number of moles, we use the equation:

Molar mass of ice = 18 g/mol
Moles of ice = 23.64 moles
Putting values in above equation, we get:
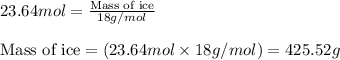
Hence, the mass of ice that would be melted is 425.52 grams