Answer : The mass percentage of isopropyl alcohol in the solution is, 59.4 %
Explanation :
To calculate the mass percentage of isopropyl alcohol in the solution, we use the equation:
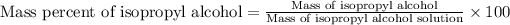
Mass of isopropyl alcohol = 6.68 g
Mass of isopropyl alcohol solution = 3.97 g
Putting values in above equation, we get:
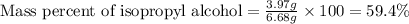
Thus, the mass percentage of isopropyl alcohol in the solution is, 59.4 %