700 J is the work done by the system.
Step-by-step explanation:
The first law of thermodynamics is that the change in internal energy of the system is equal to the net heat transfer to the system minus the complete work performed by the system.
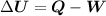
Where,
∆U – Change in internal energy
Q – Heat transfer to the system
Q – Work done
Here,
Given data:
∆U - 400 J
Q - 1100 J
We need to the work done by the system (W)
By applying the given values in the above equation, we get
400 = 1100 - W
W = 1100 - 400 = 700 J