Answer:
The molar absorptivity coefficient is,
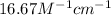 .
.
Step-by-step explanation:
Using Beer-Lambert's law :
Formula used :

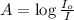
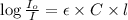
where,
A = absorbance of solution
C = concentration of solution =
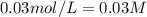
l = path length = 2 cm
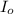 = incident light
= incident light
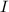 = transmitted light
= transmitted light
 = molar absorptivity coefficient = ?
= molar absorptivity coefficient = ?
A compound absorb 90 % of the light and transmit 10% of light.
Transmittance = 10% = 0.1
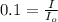
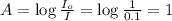
Now put all the given values in the above formula, we get the molar absorptivity coefficient.
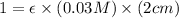
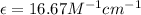
Therefore, the molar absorptivity coefficient is,
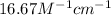 .
.