12.7 grams of copper will be produced.
Step-by-step explanation:
Copper is placed below zinc in the metal activity series. This means zinc can replace copper from its salt. Here zinc is added to the salt solution of copper sulphate. The reaction involved is
CuSO4 +Zn =ZnSO4 +Cu.
Zinc sulphate is soluble in the solution and rose red copper will be precipitated from the solution.
From the chemical equation we can see that 1 mole of zinc replaces 1 mole of copper. As no mass of zinc added is mentioned we will assume that excess of zinc is added.
Atomic mass of copper = 63.5
Atomic mass of sulphur = 32.
Atomic mass of oxygen = 16.
So molecular mass of copper sulphate =
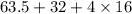 = 159.5.
= 159.5.
So, mass of 1 mole of copper sulphate is 159.5 grams.
So, in 31.9 grams of copper sulphate, number of moles of copper sulphate present =
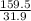 = 0.2
= 0.2
In 1 mole of copper sulphate, 1 mole of copper is present.
So in 0.2 moles of copper sulphate, 0.2 moles of copper is present.
So mass of 0.2 moles of copper =
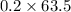 = 12.7 grams
= 12.7 grams