Answer:
2 g/mol
Step-by-step explanation:
The number of molecules contained in 1 mole of hydrogen gas is equal to Avogadro number:
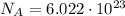
So, this is the number of molecules in 1 mole.
The mass of 1 molecule of hydrogen (which is a diatomic molecule) is equal approximately to the mass of two protons:

Therefore, the total mass of 1 mole of hydrogen molecules is equal to the mass of 1 molecule times the number of molecules:

Which corresponds to 2 g/mol