Answer:
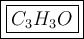
Step-by-step explanation:
1. Calculate the mass of C in 1.24 g of CO₂
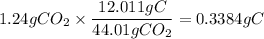
2. Calculate the mass of H in 0.255g of H₂O
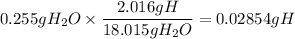
3. Calculate the mass of O by difference
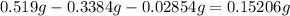
4. Convert every mass to number of moles:
- C: 0.3384g / (12.011g/mol) = 0.02817 mol
- H: 0.02854g / (1.008g/mol) = 0.02831 mol
- O: 0.15206g / (15.999g/mol) = 0.009504 mol
5. Divide every number of moles by the least number of moles (0.009504):
- C: 0.02817/0.009504 = 2.96 ≈ 3
- H: 0.02831 / 0.009504 ≈ 3
- O: 0.009504 / 0.009504 = 1
6. Those numbers are the respective subscripts in the empirical formula:
