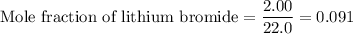Answer:

Step-by-step explanation:
The mole fraction of a component in a mixture is the number of moles of the component divided by the total number of moles of all the components, multiplied by 100.
The symbol X is used to represent the mole fraction.
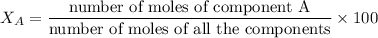
1. Convert masses in grams to number of moles
Formula:
- number of moles = mass in grams / molar mass
a) Lithium bromide
- Chemical Formula: LiBr
- Molar mass: 86.845 g/mol
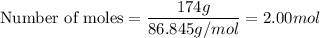
b) Water
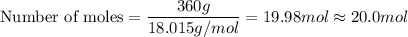
c) Total number of moles = 2.00 mol + 20.0 mol = 22.0 mol
2. Mole fraction of lithium bromide