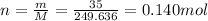Answer:
1) 0.428 mol
Here the compound that we have is
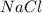 .
.
We know that:
- Molar mass of sodium (
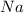 ):
):
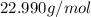
- Molar mass of chlorine (
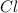 ):
):

Since the ratio between
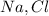 in the compound is 1:1, the molar mass of the
in the compound is 1:1, the molar mass of the
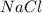 is:
is:

Here the mass of the compound is
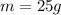
Therefore, the number of moles is:
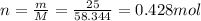
2) 1.27 mol
The compound in this problem is
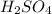
The molar mass of each element is:
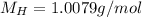 (hydrogen)
(hydrogen)
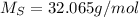 (solphur)
(solphur)
 (oxygen)
(oxygen)
In this compound, we have 2 atoms of hydrogen, 1 atom of solphur and 4 atoms of oxygen, therefore the total molar mass is:
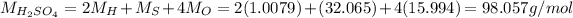
Here the mass of the compound is
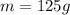
So the number of moles is
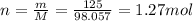
3) 0.633 mol
The compound in this problem is
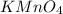
The molar mass of each element is:
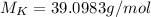 (potassium)
(potassium)
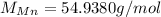 (manganese)
(manganese)
 (oxygen)
(oxygen)
Here we have 1 atom of potassium, 1 atom of manganese and 4 atoms of oxygen, so the total molar mass of the compound is
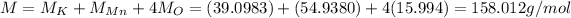
Here the mass of the compound is
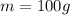
So the number of moles is
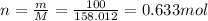
4) 0.993 mol
The compound in this problem is
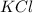
The molar mass of each element is:
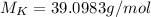 (potassium)
(potassium)
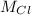 =
=
 (chlorine)
(chlorine)
So, the total molar mass of the compound is:
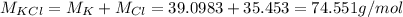
The mass of the compound here is
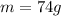
Therefore, the number of moles is
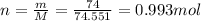
5) 0.140 mol
The compound in this problem is
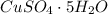
The molar mass of each element is:
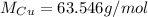 (copper)
(copper)
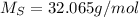 (solphur)
(solphur)
 (oxygen)
(oxygen)
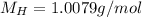 (hydrogen)
(hydrogen)
Here we have 1 atom of copper, 1 of solphure, (4+5=9) of oxygen, and (5*2=10) atoms of hydrogen, so the total molar mass is
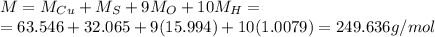
Here the mass of the compound is
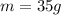
So, the number of moles is