Answer:
- He must use 50g of the 12% solution and 30 g of the 20% solution.
Step-by-step explanation:
Call x the amount of 12% boric acid solution to be used.
- The content of acid of that is: 0.12x
Since he wants to make 80 grams of solution, the amount of 20% boric acid solution to be used is 80 - x.
- The content of acid of that is{ 0.20(80 - x).
The final solution is 15% concentrated.
- The content of boric acid of that is 0.15 × 80 g.
Now you have can write your equation:
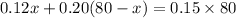
Solve:
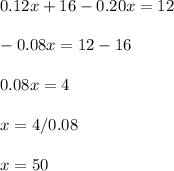
That is 50 grams of the 12% solution of boric acid.
Calculate the amount of the 20% solution of boric acid:
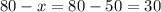
That is 30 grams.
Then, he must use 50g of the 12% solution and 30 g of the 20% solution.