Answer:
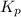 for the reaction is 3.45
for the reaction is 3.45
Step-by-step explanation:
The balanced chemical reaction is:
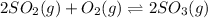
At eqm. 0.562 atm 0.101 atm 0.332 atm
As we are given that:
The expression of
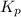 for above equation follows:
for above equation follows:
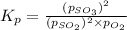
Putting values in above equation, we get:
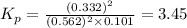
The value of
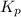 for the reaction is 3.45
for the reaction is 3.45