Answer:
The relation is linear between
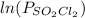 and time as given in the graph attached thus the reaction is first order wrt
and time as given in the graph attached thus the reaction is first order wrt
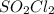 .
.
Step-by-step explanation:
As the reaction is given as
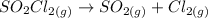
This indicates that 1 mole of
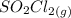 dissociates into exactly one mole of
dissociates into exactly one mole of
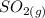 and
and
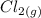 .
.
By indicating the degree of dissociation as ζ and solving for the pressure gives
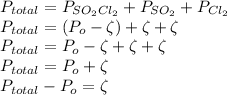
Now by calculation of degree of dissociation ζ and thus pressure of
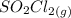 is that calculated as
is that calculated as
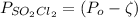
For the data given the calculation is as below
t P(total) Dissociation(ζ) P(SO2CL2) ln(P(SO2CL2))
P(total)-11.07 11.07-ζ
0 11.07 0 11.07 2.4042
3 14.79 3.72 7.35 1.9947
6 17.26 6.19 4.88 1.5851
9 18.9 7.83 3.24 1.1756
12 19.99 8.92 2.15 0.7655
15 20.71 9.64 1.43 0.3577
For the estimation of the degree the relation between
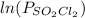 vs time is to be plotted, if the relation is linear it is a first order reaction, if the relation is not linear than it is a higher order reaction. The relation is linear as given in the graph attached thus the reaction is first order wrt SO2Cl2.
vs time is to be plotted, if the relation is linear it is a first order reaction, if the relation is not linear than it is a higher order reaction. The relation is linear as given in the graph attached thus the reaction is first order wrt SO2Cl2.