Answer:
17.85% is the organic content of the carrot tissue.
Step-by-step explanation:
Percentage of water is carrot sample = 79%
Mass of the sample = M
Mass of the water is sample = 79% of M = 0.79M
Percentage of dried carrot sample = 100%-79%=21%
Mass of the carrot sample after drying = 8 g
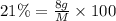
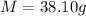
Mass of ash =- 1.2 g
Mass of organic matter = 8 g - 1.2 g = 6.8 g
Percentage of organic matter ;
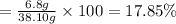
17.85% is the organic content of the carrot tissue.