Answer:
17.2 minutes is the value of the turnover number.
Step-by-step explanation:
Using Michaelis-Menten equation:
![V = V_(max)* ([S])/( (Km + [S]))](https://img.qammunity.org/2021/formulas/chemistry/college/htylyoyc0o3ns4lspuu3zy2q5b0uk7y045.png)
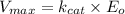
Where :
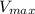 = max rate velocity
= max rate velocity
[S] = substrate concentration
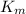 = Michaelis-Menten constant
= Michaelis-Menten constant
V = reaction rate
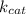 = catalytic rate constant
= catalytic rate constant
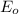 = initial enzyme concentration
= initial enzyme concentration
We have :
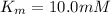
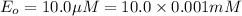
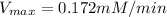
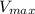 is the rate is obtained when all enzyme is bonded to the substrate.
is the rate is obtained when all enzyme is bonded to the substrate.
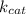 is termed as the turnover number.
is termed as the turnover number.
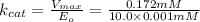
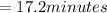
17.2 minutes is the value of the turnover number.