Answer: The pH and pOH of the solution is 1 and 13 respectively and the solution is acidic in nature.
Step-by-step explanation:
There are three types of solution: acidic, basic and neutral
To determine the type of solution, we look at the pH values.
- The pH range of acidic solution is 0 to 6.9
- The pH range of basic solution is 7.1 to 14
- The pH of neutral solution is 7.
We are given:
Concentration of HI = 0.100 M
1 mole of HI produces 1 mole of hydrogen ions and 1 mole of iodide ions
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
We are given:
![[H^+]=0.100M](https://img.qammunity.org/2021/formulas/chemistry/college/8o9l1ai90y755huoo4x1dzwqowi1c7j4er.png)
Putting values in above equation, we get:
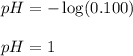
To calculate the pOH of the solution, we use the equation:
pH + pOH = 14
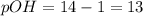
Hence, the pH and pOH of the solution is 1 and 13 respectively and the solution is acidic in nature.