Answer: The ratio of
![([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/zu05l2hw1fqjqd9x2ofm3ovlcqytwbmx51.png) is 0.1
is 0.1
Step-by-step explanation:
The chemical equation for the dissociation of monoprotic weak acid (formic acid) follows:
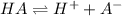
To calculate the pH of acidic buffer, we use the equation given by Henderson Hasselbalch:
![pH=pK_a+\log(([salt])/([acid]))](https://img.qammunity.org/2021/formulas/biology/college/6usxe642bp3w274zbcv30her0kcessu95f.png)
![pH=pK_a+\log(([A^-])/([HA]))](https://img.qammunity.org/2021/formulas/chemistry/college/rm48zntdi8ffuy0zd7czbj5vut380xsr21.png)
We are given:
 = negative logarithm of acid dissociation constant of methanoic acid = 3.75
= negative logarithm of acid dissociation constant of methanoic acid = 3.75
![([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/zu05l2hw1fqjqd9x2ofm3ovlcqytwbmx51.png) = ratio of concentration of salt and acid
= ratio of concentration of salt and acid
pH = 2.75
Putting values in above equation, we get:
![2.75=3.75+\log(([A^-])/([HA]))\\\\([A^-])/([HA])=0.1](https://img.qammunity.org/2021/formulas/chemistry/college/wqu3flkecpqrmjk0djeg855hnzzbw2ag5l.png)
Hence, the ratio of
![([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/zu05l2hw1fqjqd9x2ofm3ovlcqytwbmx51.png) is 0.1
is 0.1