Answer:
ΔV=2.481 L
Step-by-step explanation:
As we know that
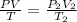
Always use temperature in Kelvin and Pressure in Atm
Given Data
P₁=1.03 atm
P₂=225 torr =(225/760)=0.296 atm
V₁=1.30
T=17⁰C=290.15 K
T₂=-31⁰C=242.15 K
Substitute the given values
So
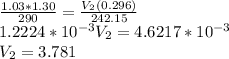
You have to find the difference of two volumes:
So
ΔV=3.781 - 1.30
ΔV=2.481 L