Answer : The correct option is, (B) bimolecular
Explanation :
Molecularity : It is defined as the total number of reactant molecules taking part in the balanced equation of a reaction. It is a theoretical concept.
The given chemical reaction are,
Step (1) :
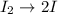
Step (2) :
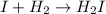
Step (3) :
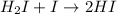
The number of reactants molecules taking part in the balanced equation of a reaction 2 are,
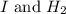 .
.
In this reaction, 1 'I' molecules reacts with the 1
 molecule.
molecule.
Total number of reactant molecule = 1 + 1 = 2
The molecularity of the reaction is 2 that means, the elementary reaction is bimolecular.
Hence, the correct option is, (B) bimolecular