The question is incomplete. the complete question is:
A chemist measures the energy change during the following reaction:
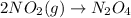
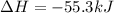 1. This reaction is:______. a. endothermic. b. exothermic.
1. This reaction is:______. a. endothermic. b. exothermic.
2. Suppose 70.1 g of NO2 react. Will any heat be released or absorbed? A. Yes, absorbed. B. Yes, released. C. No.
3. If you said heat will be released or absorbed, calculate how much heat will be released or absorbed?
Answer:1. b. exothermic
2. Yes , released
3. 42.0 kJ
Step-by-step explanation:
1. Endothermic reactions are those in which heat is absorbed by the system and exothermic reactions are those in which heat is released by the system.
 for Endothermic reaction is positive and
for Endothermic reaction is positive and
 for Exothermic reaction is negative.
for Exothermic reaction is negative.
2.
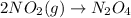
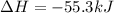
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
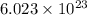 of particles.
of particles.
To calculate the moles, we use the equation:
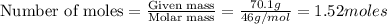
2 moles of
 reacts, energy released = 55.3 kJ
reacts, energy released = 55.3 kJ
1.52 moles of
 reacts, energy released =
reacts, energy released =
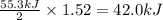
Thus 42.0 kJ heat will be released when 70.1 g of
 react.
react.