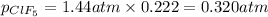The question is incomplete, here is the complete question:
A 5.00 L tank at 4.19°C is filled with 16.7 g of boron trifluoride gas and 9.15 g of chlorine pentafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits.
a. mole fraction of boron trifluoride:
b. partial pressure of boron trifluoride:
c. mole fraction of chlorine pentafluoride:
d. partial pressure of chlorine pentafluoride:
e. Total pressure in tank:
Answer:
For a: Mole fraction of boron trifluoride is 0.778
For b: Partial pressure of boron trifluoride is 1.12 atm
For c: Mole fraction of chlorine pentafluoride is 0.222
For d: Partial pressure of chlorine pentafluoride is 0.320 atm
For e: Total pressure in tank is 1.44 atm
Step-by-step explanation:
To calculate the number of moles, we use the equation:
 .....(1)
.....(1)
Given mass of boron trifluoride = 16.7 g
Molar mass of boron trifluoride = 67.8 g/mol
Putting values in equation 1, we get:
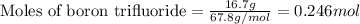
- For chlorine pentafluoride:
Given mass of chlorine pentafluoride = 9.15 g
Molar mass of chlorine pentafluoride = 130.5 g/mol
Putting values in equation 1, we get:
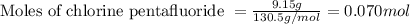
To calculate the pressure of the tank, we use the equation given by ideal gas which follows:

where,
P = pressure of the tank = ?
V = Volume of the tank = 5.00 L
T = Temperature of the gas =
![4.19^oC=[4.19+273]K=277.19K](https://img.qammunity.org/2021/formulas/chemistry/college/sch1h1yibjsqk8p5o2ubpba6usuckqgmas.png)
R = Gas constant =
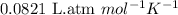
n = Total number of moles of the tank = [0.246 + 0.070] = 0.316 moles
Putting values in above equation, we get:
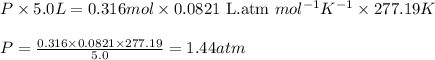
Mole fraction of a substance is given by:
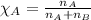 .......(2)
.......(2)
To calculate the partial pressure, we use the equation given by Raoult's law, which is:
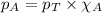 .....(3)
.....(3)
Moles of boron trifluoride = 0.246 moles
Total moles = 0.316 moles
Putting values in equation 2, we get:
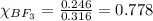
We are given:
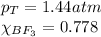
Putting values in equation 3, we get:
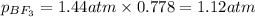
- For chlorine pentafluoride:
Moles of chlorine pentafluoride = 0.070 moles
Total moles = 0.316 moles
Putting values in equation 2, we get:
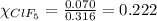
We are given:
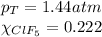
Putting values in equation 3, we get: