Answer:
This is not true
Step-by-step explanation:
The limiting reactant is described as the reactant in short supply. This indicates that a limiting reactant does not depend on the amount of the reactant given.
The extent to which it is used up in the reaction determines whether a reactant is limiting or not.
For example;
2H₂ + O₂ → 2H₂O
The equation above is balanced.
Lets say 20mole of H₂ reacted with 20mole of O₂
According to the statement in the problem, since the equation is balanced and we have equal number of reactants, then there is no limiting reactant.
This is false
2 mole of H₂ reacted with 1 mole of O₂
20 mole of H₂ will require
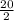 moles of O₂ = 10 mole of O₂
moles of O₂ = 10 mole of O₂
But we were given 20 moles of O₂. We see that O₂ is in excess and H₂ is the limiting reactant.