Here is the full and correct question
In the "Methode Champenoise", grape juice is fermented in a wine bottle to produce sparkling wine. The reaction is:
C₆H₁₂O₆(aq) -----------> 2C₂H₅OH(aq) + 2CO₂(g)
Fermentation of 826 mL grape juice (density is 1.0 g/cm³) is allowed to take place in a bottle with a total volume of 885 mL until 19% by volume is ethanol(C₂H₅OH).
Assuming that:
CO₂ obeys Henry's Law, calculate the partial pressure of CO₂ in the gas phase and the Solubility of CO₂ in the wine at 25°C
The Henry Law Constant for CO₂ is 3.1 × 10⁻² mol/L. atm at 25°C with Henry's Law in the form C = KP;
where:
C = concentration of the gas in mol/L.
(The solubility of ethanol is 0.79 g/cm³)
Answer:
Partial Pressure of CO₂ in the gas phase is 104.84 atm
Solubility of CO₂ in the wine at 25°C is 3.25004 M
Step-by-step explanation:
The equation for the reaction is given as:
C₆H₁₂O₆(aq) -----------> 2C₂H₅OH(aq) + 2CO₂(g)
Given that:
The total volume = 885 mL
Volume of ethanol is by 19% of the total volume = 0.19 × 885
Density = 0.79
density =

the mass of ethanol can be calculated as = 0.79 × 0.19 × 885 = 132.84 g
number of moles of ethanol =
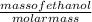
molar mass of ethanol = 46.07
∴
number of moles of ethanol =
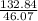
= 2.8834 mole
Henry's Law posits that the solubility of a gas is directly proportional to the partial pressure of the gas above the solution.
NOW, Assuming that CO₂ obeys Henry's Law;
then numbers of moles of ethanol = numbers of mole of CO₂
So, molar mass of CO₂ = 44.01
then mass of CO₂ = number of moles of CO₂ × molar mass
mass of CO₂ = 2.8834 mole × 44.01
mass of CO₂ = 126.90 g
Since total volume = 885 mL = 0.885 m
Concentration of CO₂ =
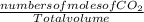
Concentration of CO₂ =
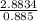
Concentration of CO₂ = 3.258 M
C =

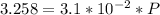
P =
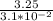
P = 104.84 atm
∴ the partial pressure of in the gas phase = 104.84 atm
b)
Solubility of ethanol in the wine =
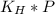
Solubility of ethanol in the wine =
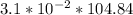
Solubility of ethanol in the wine = 3.25004 M