The question is incomplete. The complete question is:
A certain molecular compound M has a solubility in acetone of 0.737g/mL at 10.°C .Calculate the mass of M that's dissolved in 9.0 L of a saturated solution of M in acetone at this temperature.
Be sure your answer has the correct unit symbol and number of significant digits.
Answer: 6633 g of M is dissolved in 9.0 L of saturated solution in hexane at this temperature.
Step-by-step explanation:
Given : 0.737g of solute is present in 1ml of solution
i.e 1ml of solution contains = 0.737 g of solute
Thus 9L or 9000ml of solution contains=
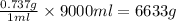
Thus 6633 g of M is dissolved in 9.0 L of saturated solution in hexane at this temperature.