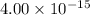Answer:
The fraction of collision is
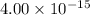
Step-by-step explanation:
Given that,
Temperature = 47°C
Activation energy = 88.20 KJ/mol
From Arrhenius equation,
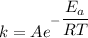
Here,
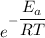 =fraction of collision
=fraction of collision
We need to calculate the fraction of collisions
Using formula of fraction of collisions
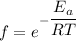
Where f = fraction of collision
E = activation energy
R = gas constant
T = temperature
Put the value into the formula
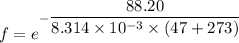
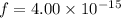
Hence, The fraction of collision is